The rare, silvery white element named tungsten (W) is a metal of superlatives. Its melting point is the highest of any pure metal. Its boiling point approaches surface temperatures of the sun. Its tensile strength is the highest of any metal under extreme temperatures. And its coefficient of thermal expansion is the lowest of any metal (meaning, it undergoes minimal expansion when heated).
Why such extraordinary properties? The answer lies in what is called electron bonding. Each tungsten atom has 74 electrons encircling its densely packed core. Four of these electrons, given the catchy name of 5d, form a powerful bond with other 5d electrons in nearby tungsten atoms. Thus, it takes tremendous thermal energy to separate the atoms and turn solid tungsten into molten metal.
The main ores of tungsten are scheelite (named after Karl Scheele, co-discoverer of tungsten) and wolframite. Adex's Mount Pleasant deposit contains significant quantities of wolframite.
Main Uses. The primary use of tungsten is to produce various hardmetals containing tungsten monocarbide, which rivals diamond in hardness. Tungsten added to steel increases metal strength, tensile resilience, hardness and flexibility. The mining, drilling, construction, aeronautical and metalworking sectors all depend on tungsten-bearing metals for tools and equipment that function safely at high speeds and over a wide temperature range.
Tungsten is a mainstay of microwave ovens and many electric light bulbs. Look inside an incandescent bulb, and you'll see a thin tungsten wire designed to withstand the searing heat of electrical currents. The growing popularity of tungsten wedding rings creates a whimsical but apt metaphor, mirroring the strong bond between tungsten atoms in nature.
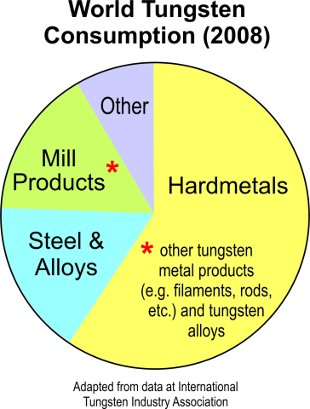
Outlook. Tungsten remains indispensible in many applications, even though molybdenum (lighter in weight) and copper have come to replace it in some industrial products. Research is ongoing to develop incandescent light bulbs that use tungsten wire which are more energy efficient—an initiative spurred by the legislated ban on low-efficiency bulbs in several countries.
The following links give further information on tungsten uses, production, technological advances and market data.
Mineral Commodity Summary for 2010 (Tungsten) by United States Geological Survey.
Mineral Information (2010): Tungsten by United States Geological Survey.
Canadian Minerals Yearbook, 2007: Tungsten by Natural Resources Canada.
International Tungsten Industry Association. Provides comprehensive information on the uses, history, chemistry, metallurgy, markets and global production of tungsten, as well as a research database (for members only) of the recent tungsten-based technologies.

